Articles/News

The power of oceans: how much CO2 are they able to absorb
Ocean Carbon Uptake. This is the expression used by scientists to explain how much CO2 emitted is absorbed by Oceans. Knowing the rate at which the oceans absorb carbon pollution is a key to understanding how fast climate change will occur. This is what a group of scientists, led by Tim DeVries is trying to do. On the other hand, understanding the impact of CO2 ocean uptake have in terms of ocean acidification is important too. Let’s see how Ocean Carbon Uptake work and how it is measured.
(by Dario Ruggiero)
“As atmospheric CO2 increases,
the interaction with the surface ocean will change
the chemistry of the seawater resulting in ocean acidification”
How does it work?
Air-sea gas exchange is a physio-chemical process, primarily controlled by the air-sea difference in gas concentrations and the exchange coefficient (which determines how quickly a molecule of gas can move across the ocean-atmosphere boundary). Whenever the partial pressure of a gas is increased in the atmosphere over a body of water, the gas will diffuse into that water until the partial pressures across the air-water interface are equilibrated. As atmospheric CO2 increases, the interaction with the surface ocean will change the chemistry of the seawater resulting in ocean acidification.
“Ocean acidification refers to a reduction in the pH
of the ocean over an extended period of time…
The entire food web may also be at risk….”
The downside effect: Ocean Acidification
Fundamental changes in seawater chemistry are occurring throughout the world’s oceans. Since the beginning of the industrial revolution, the release of carbon dioxide (CO2) from humankind’s industrial and agricultural activities has increased the amount of CO2 in the atmosphere. The ocean absorbs about a quarter of the CO2 we release into the atmosphere every year, so as atmospheric CO2 levels increase, so do the levels in the ocean. Initially, many scientists focused on the benefits of the ocean removing this greenhouse gas from the atmosphere. However, decades of ocean observations now show that there is also a downside — the CO2 absorbed by the ocean is changing the chemistry of the seawater, a process called Ocean Acidification. – Source: NOAA – PMEL program
Ocean acidification refers to a reduction in the pH of the ocean over an extended period of time. These changes in ocean chemistry can affect the behavior of non-calcifying organisms as well. Certain fish’s ability to detect predators is decreased in more acidic waters. When these organisms are at risk, the entire food web may also be at risk.
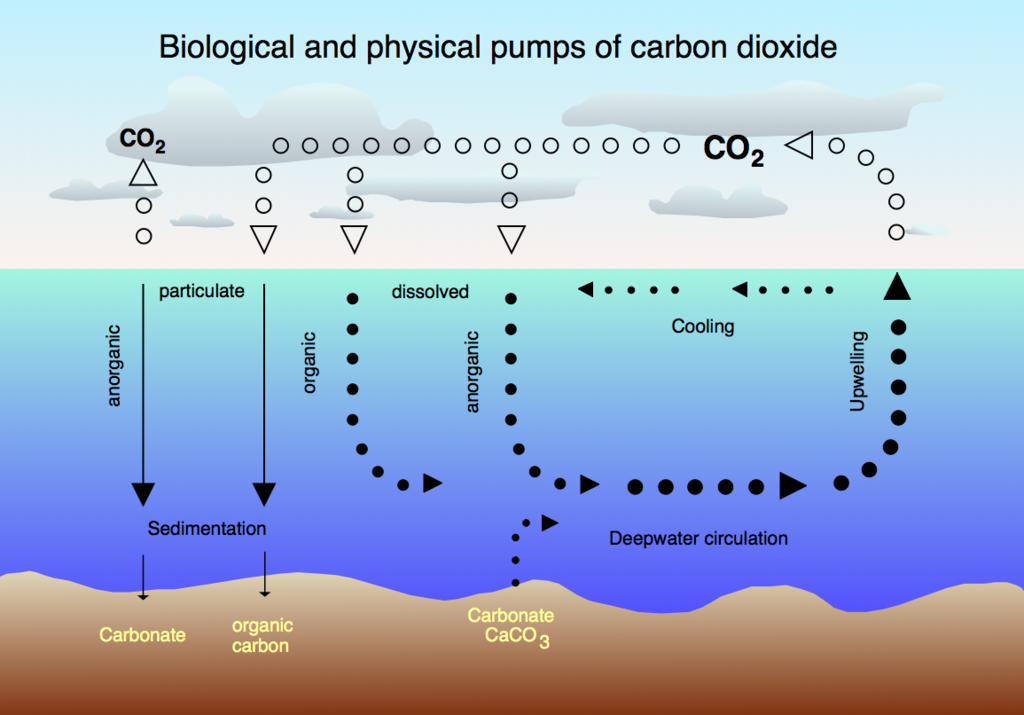
As the oceans warm, they will become
less and less capable of taking up carbon dioxide.
As a result, more of our carbon pollution
will stay in the atmosphere, exacerbating global warming
How much is ocean uptake rate
The process of absorption is not simple – the amount of carbon dioxide that the ocean can hold depends on the ocean temperatures: colder waters can absorb more carbon; warmer waters can absorb less. So, a prevailing scientific view is that as the oceans warm, they will become less and less capable of taking up carbon dioxide. As a result, more of our carbon pollution will stay in the atmosphere, exacerbating global warming. But it’s clear that at least for now, the oceans are doing us a tremendous favor by absorbing large amounts of carbon pollution.
How much carbon dioxide is being absorbed by the oceans is an active area of research. In particular, scientists are closely watching the oceans to see if their ability to absorb is changing over time. Such a study is the topic of a very recent paper published in the journal Nature. The authors studied recent ocean carbon dioxide uptake and in particular the mystery of why it appears the oceans are actually becoming more absorbing.
Why oceans are actually becoming more absorbing?
The authors describe a slowdown in a major ocean current called the overturning circulation. That circulation brings dense salty water from the surface to the depths of the ocean while simultaneously bringing colder but less salty and dense water upwards. Why is this important current slowing down? It’s possible that global warming is a culprit.
In fact, a slowdown of the current is a prediction of global warming. As the Earth warms, ice melt – especially near the Arctic – flows into the oceans. That meltwater has less salt and therefore is less dense than the surrounding waters. In a certain sense, the freshwater can block the overturning circulation, making it difficult for water near the surface to sink to the ocean depths. But it is also possible that the circulation just changes naturally. To conduct the study, the scientists used what are called ‘tracer data.’ Tracers are chemicals in the ocean that are used to track ocean currents.
The authors explained that ocean circulation currents tend to bring deep carbon-rich fluids to the surface where it can then escape to the atmosphere. When the ocean circulation gets weaker, less of this carbon rich water is at the surface so the transfer of carbon to the atmosphere is less intense.
Conclusions
The findings are a bit counter-intuitive because many scientists expect the deeper, colder waters to be able to hold more carbon dioxide. The authors of this study remind us that the transfer of carbon dioxide between the atmosphere and the oceans is a two-way street – carbon can go either way. Changing the emission of carbon dioxide from the ocean or the ability of the ocean to absorb are two ways to alter the overall carbon uptake. So, we can be grateful that the oceans are doing us a service by reducing the amount of carbon dioxide in the atmosphere. It buys us more time to reduce our dependency on fossil fuels. On the other hand, it isn’t a free gift. The increased carbon uptake by the ocean means that the ocean waters will become acidic more rapidly than they otherwise would. This acidification threatens many base-level components of the food chain.
What’s the lesson from this process? 1) The fact that oceans absorb CO2 is not a valid reason to still emit CO2 in the atmosphere; 2) Especially considering the fact that the more CO2 oceans absorbs, the more acid they become.

Dario Ruggiero
0
Tags :